Summary: Researchers have developed a new solid polymer electrolyte using a vertically-aligned aramid nanofiber (ANF) aerogel framework. This innovative design significantly improves the performance of solid-state lithium-metal batteries (SSLMBs).

Solid-state lithium-metal batteries are promising for next-generation energy storage due to their high energy density and safety. The solid electrolyte is a key component. While polymer electrolytes offer advantages like good flexibility, they often suffer from low ionic conductivity at room temperature.
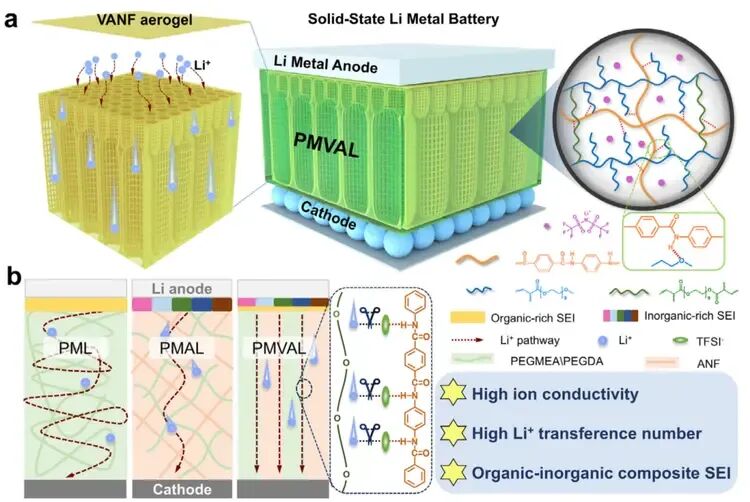
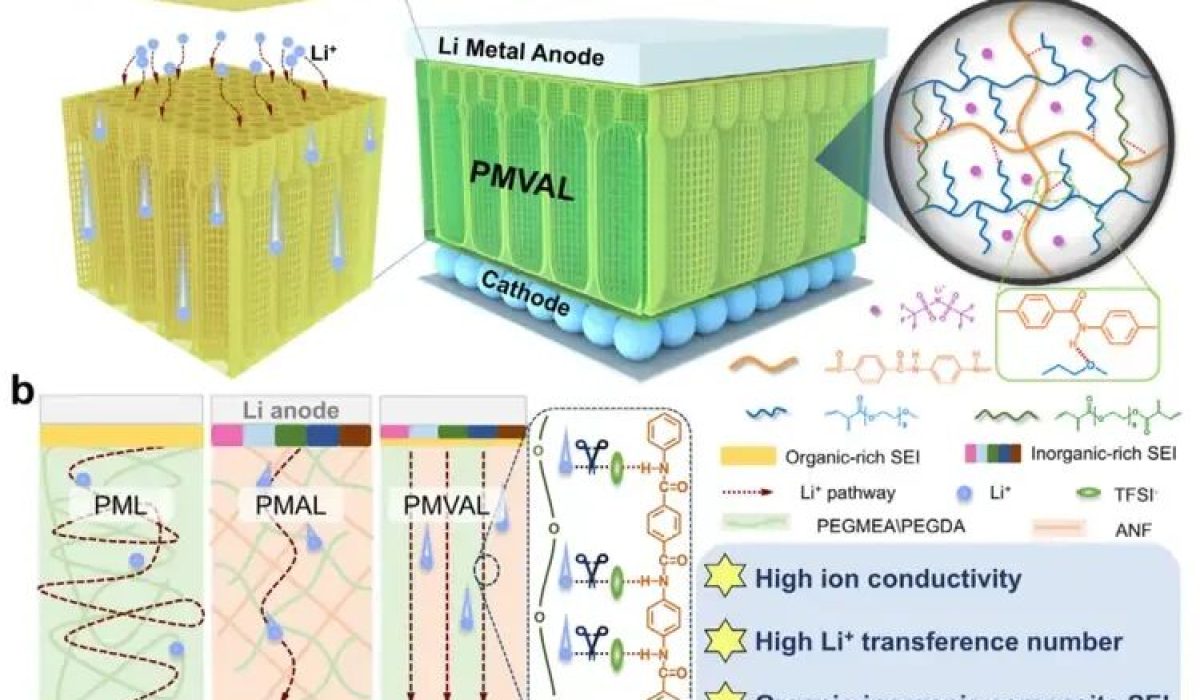
Figure 1 a) Structure of PMVAL: Shows the multi-layered composition of the electrolyte.b) Ion Transport Comparison: Compares the lithium-ion pathways in three different electrolytes (PML, PMAL, PMVAL). It highlights how the unique vertical channels in the aramid nanofiber framework of PMVAL create direct highways for ions. This design enhances ionic conductivity, suppresses lithium dendrite growth, and improves overall battery performance.
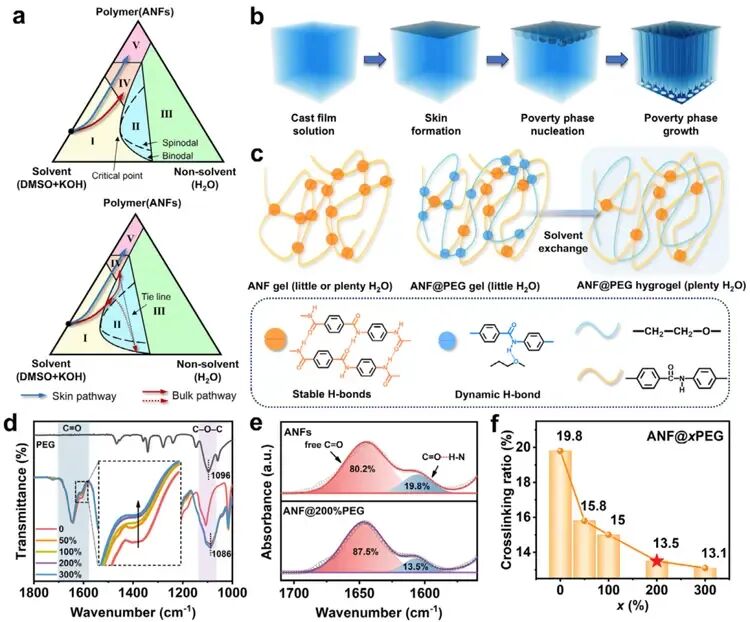
Figure 2. Mechanism for Creating Aligned Pores in Aramid Nanofiber (ANF) Aeroge a) Phase Diagram: A diagram showing how the ANF solution transforms.b) Pore Formation Process: The step-by-step process of building the aligned pore structure.c) Role of PEG: Polyethylene glycol (PEG) adds dynamic hydrogen bonds, which helps control the assembly of ANFs and the final structure.d)FTIR Spectra of ANF@xPEG and PEG e) FTIR Analysis: These graphs (Fourier-transform infrared spectroscopy) show the chemical interactions, confirming how PEG affects the ANFs.f) Crosslinking Ratio: A chart showing how the amount of PEG changes the level of linking between ANFs.
To solve this, a research team created an electrolyte named PMVAL. Its core is a special aerogel made from aramid nanofibers, fabricated using a non-solvent induced phase separation (NIPS) technique. This process creates a structure with vertically aligned channels, like tiny highways for lithium ions.
Figure 3 shows the dynamic formation process and the final multi-layered pore structure of the VANF gel, using both optical and scanning electron microscopy (SEM) images.
The aerogel has a clear three-layer structure:
- Layer 1: A dense surface skin.
- Layer 2: An array of small pores with a diameter of about 30 μm.
- Layer 3: Larger pore channels, about 60 μm in diameter, extending to the bottom of the membrane.
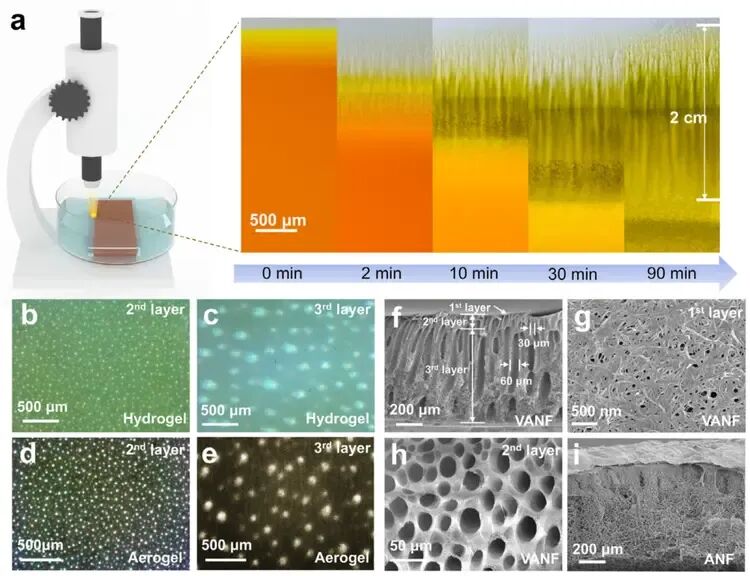
Figure 3. Dynamic Formation Process and Morphological Characterization of VANF Gel
- a) Schematic diagram and optical microscopy (OM) images of the phase inversion process in the ANF solution.
- b-c) OM images of Layer 2 and Layer 3 of the VANF hydrogel.
- d-e) OM images of Layer 2 and Layer 3 of the VANF aerogel.
- f-h) Scanning electron microscopy (SEM) images of the VANF aerogel: (f) cross-sectional view, (g) Layer 1, (h) Layer 2.
- i) Cross-sectional SEM image of the ANF aerogel.
In contrast, an ANF aerogel prepared without the NIPS method shows a uniform, sponge-like structure lacking these aligned, directional pores.
The PMVAL electrolyte was fabricated by infiltrating the polymer precursor into the VANF aerogel, followed by thermal polymerization. Figure 4 shows its fundamental and electrochemical properties:
- The DSC curve indicates that PMVAL has a lower glass transition temperature (-55.9°C), suggesting enhanced chain segment mobility.
- Tensile tests reveal a Young’s modulus of 0.55 MPa, indicating a good balance of mechanical strength and flexibility.
- Raman spectroscopy analysis shows that PMVAL achieves the highest degree of lithium salt dissociation (93.5%). Its ionic conductivity and lithium-ion transference number (0.41) are significantly superior to those of the comparative samples.
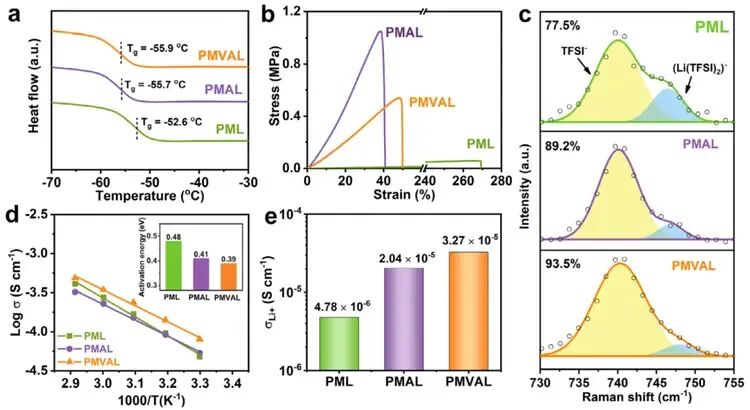
Figure 4. Fundamental and Electrochemical Properties of PMVAL
- a) Differential scanning calorimetry (DSC) curves of PML, PMAL, and PMVAL.
- b) Stress-strain curves.
- c) Raman spectra.
- d) Arrhenius plots (the inset shows the corresponding activation energy, Ea).
- e) Li⁺ conductivity (σLi⁺) at 30°C.
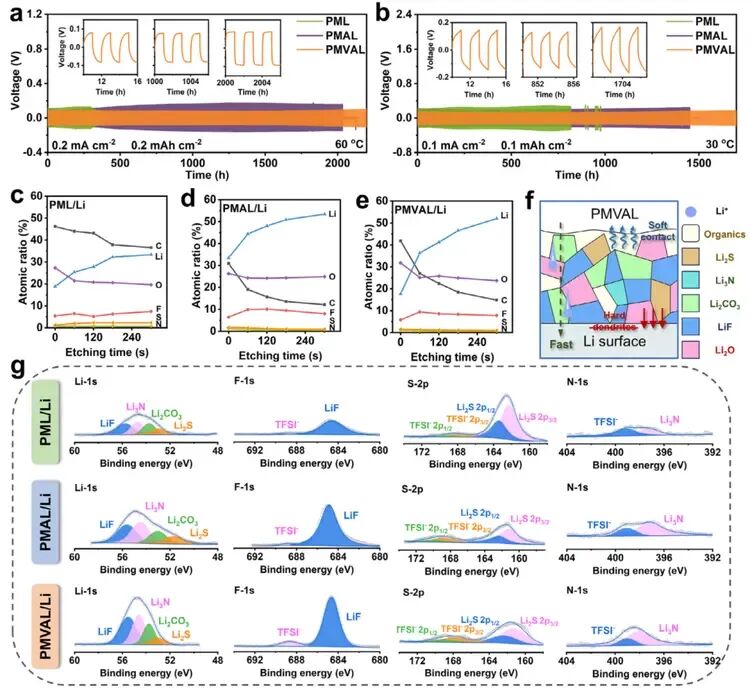
Figure 5. Symmetric Cell Performance and Interface Analysis for PML, PMAL, and PMVAL
- a) Galvanostatic cycling profiles of Li||Li symmetric cells at 0.2 mA cm⁻², 0.2 mAh cm⁻², and 60°C (Insets show the voltage profiles at 10, 1000, and 2000 hours).
- b) Galvanostatic cycling profiles of Li||Li symmetric cells at 0.1 mA cm⁻², 0.1 mAh cm⁻², and 30°C (Insets show the voltage profiles at 10, 850, and 1700 hours).
- c-e) Atomic ratio distribution vs. sputtering time for the (c) PML/Li, (d) PMAL/Li, and (e) PMVAL/Li interfaces after 100 cycles at 0.1 mA cm⁻², 0.1 mAh cm⁻², and 30°C.
- f) Schematic diagram of the composition at the PMVAL/Li interface.
- g) High-resolution Li 1s, F 1s, S 2p, and N 1s XPS spectra of the PML/Li, PMAL/Li, and PMVAL/Li interfaces after 120 seconds of Ar⁺ sputtering, following 100 cycles at 0.1 mA cm⁻², 0.1 mAh cm⁻², and 30°C.
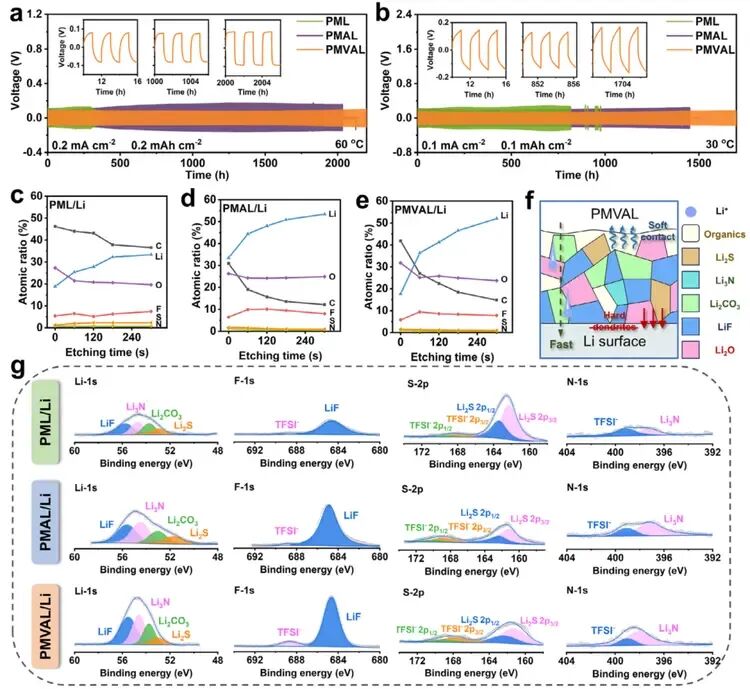
Figure 6 demonstrates the practical performance of PMVAL in full cells. The Li|PMVAL|LFP battery maintained 55% capacity after 5000 cycles at 1C and 60°C, and also cycled stably for 150 cycles under high load conditions (8 mg cm⁻²). At 30°C, it achieved an 85% capacity retention after 1000 cycles at 0.5C, along with excellent high-rate performance. When paired with a high-voltage NCM811 cathode, PMVAL similarly exhibited good cycling stability. The flexible pouch cell continued to power an LED normally even after repeated folding, puncturing, and cutting, showing no thermal runaway or short circuit, which demonstrates extremely high safety and practical potential.
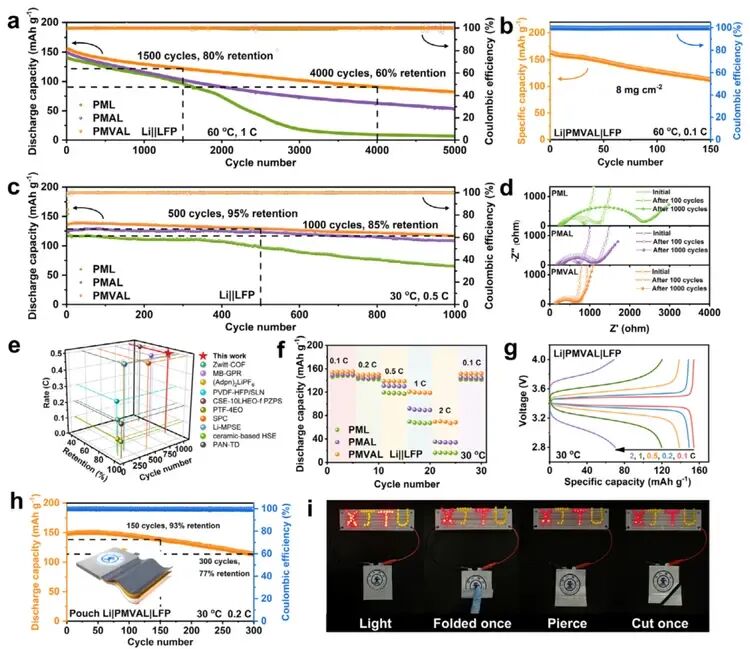
Figure 6. Practical Performance of PML, PMAL, and PMVAL in Full Cells
a) Long-term cycling performance of Li||LiFePO₄ (LFP) batteries at 1C and 60°C.
b) Cycling performance of a high-loading Li|PMVAL|LFP battery at 0.1C and 60°C (LFP loading: 8.0 mg cm⁻²).
c) Long-term cycling performance of Li||LFP batteries at 0.5C and 30°C.
d) Electrochemical impedance spectroscopy (EIS) curves of Li||LFP batteries after 0, 100, and 1000 cycles.
e) Comparison of the cycling performance of the Li|PMVAL|LFP battery at room temperature (RT) with recent literature.
f) Rate capability of Li||LFP batteries at 30°C.
g) Voltage profiles of the Li|PMVAL|LFP battery at different current rates.
h) Cycling stability of a Li|PMVAL|LFP pouch cell (Inset: schematic diagram of the pouch cell structure).
i) Optical images of a flexible Li|PMVAL|LFP pouch cell powering an XJTU LED series under different states and destructive conditions at room temperature.
Key Benefits of the ANF Aerogel Framework:
- Faster Ion Transport: The vertical channels help lithium ions move quickly and directly. The PMVAL electrolyte achieved a high ionic conductivity.
- Suppresses Dendrites: It helps form a stable protective layer (SEI) at the lithium metal interface, preventing the growth of needle-like lithium dendrites that can short-circuit batteries.
- Excellent Stability: Batteries using PMVAL showed outstanding long-life performance, cycling over 5000 times at 60°C and 1000 times at 30°C with high efficiency.
- High Safety: Flexible pouch cells made with this electrolyte remained stable and functional even when bent, punctured, or cut, demonstrating exceptional safety.
This work highlights the great potential of vertically-aligned aramid nanofiber aerogels in creating high-performance, safe solid-state batteries for future applications.