INTRODUCTION
All fibers used in polymer engineering composites can be divided into two categories: synthetic and natural fibers. Synthetic fibers are the most common. Although there are many synthetic fibers, glass, carbon and aramid fibers are the most important.
Kevlar fiber is an aromatic polyamide or aramid fiber introduced by DuPont in the early 1970s. It was the first organic fiber with sufficient tensile strength and modulus to be used in advanced composites. It has approximately five times the tensile strength of steel with a corresponding tensile modulus. Originally developed as a replacement for steel in radial tires, Kevlar is now used in a wide range of applications. It is a trade name for aramid fiber.
The U.S. Federal Trade Commission defines an aramid fiber as “a manufactured fiber in which the fiber-forming substance is a long chain synthetic polyamide in which at least 85% of the amide linkages are attached directly to two aromatic rings”.
History and Development of Kevlar
Kevlar’s history goes back to 1948, when DuPont, with its invention of nylon behind it, decided to pursue work in a broad area of fibers with unusually high thermal, elasticity, and strength properties.
The possibility of making polyaramid plastic was hypothesized in 1939. It was synthesized and identified at DuPont in 1960, but polyaramid fiber could not be produced until 1965, when Stephanie Kwolek, a chemist at DuPont, discovered a practical solvent. At about the same time, a team at Akzo, Inc., a multinational firm headquartered in Holland, independently discovered a practical solvent and applied for a patent for the manufacture of polyaramid fiber, which DuPont named Kevlar® and Akzo later (1984) named Twaron®. DuPont contested the patent. A consent decree of the International Trade Commission settled the dispute; terms of the settlement included cross-licensing but barred Akzo from marketing Twaron® in the United States until late 1990.
Kevlar 29, introduced in the early 1970s, was the first generation of bullet-resistant fibers developed by DuPont and helped to make the production of flexible, concealable body armor practical for the first time. In 1988, DuPont introduced the second generation of Kevlar fiber, known as Kevlar 129. According to DuPont, this fabric offered increased ballistic protection capabilities against high-energy rounds such as the 9mm FMJ. In 1995, Kevlar Correctional was introduced, which provides puncture-resistant technology to both law enforcement and correctional officers against puncture-type threats.
The newest addition to the Kevlar line is Kevlar Protera, which became available in 1996 by DuPont. DuPont contends that the Kevlar Protera is a high-performance fabric that allows lighter weight, more flexibility, and greater ballistic protection in a vest design due to the molecular structure of the fiber. Its tensile strength and energy-absorbing capabilities have been increased by the development of a new spinning process. Before Kevlar® was used for body armor, it was used as a substitute for steel in the manufacture of radial tires, including those designed for police cars. “Kevlar” is a registered trademark of DuPont de Nemours and Co., Inc. “Twaron” is a registered trademark of Akzo, Inc. “Vicwa” is a registered trademark of Sinoara Advanced Materials Co., Ltd.
Composition of Para-Aramid Fiber
The chemical composition of Kevlar is poly para-phenyleneterephthalamide (PPD-T) and it is more properly known as a para-aramid. It is oriented para-substituted aromatic units. Aramids belong to the family of nylons. Common nylons, such as nylon 66 do not have very good structural properties, so the para-aramid distinction is important. Aramid fibers like Meta-Aramid fiber or Para-aramid fiber, however, are ring compounds based on the structure of benzene as opposed to linear compounds used to make nylon. The aramid ring gives para-aramid fiber thermal stability, while the para structure gives it high strength and modulus. Like nylons, para-aramid fiber filaments are made by extruding the precursor through a spinneret. The rod form of the para-aramid molecules and the extrusion process makes Para-aramid fibers anisotropic–they are stronger and stiffer in the axial direction than in the transverse direction. In comparison, graphite fibers are also anisotropic, but glass fibers are isotropic.
Figure 1: Chemical composition of Para-aramid fiber
It is made from a condensation reaction of para-phenylene diamine and terephthaloyl (PPD-T) chloride. The resultant aromatic polyamide contains aromatic and amide groups which makes them rigid rod-like polymers. The rigid rod-like structure results in a high glass transition temperature and poor solubility, which makes fabrication of these polymers, by conventional drawing techniques, difficult Instead, they are melt spun from liquid crystalline polymer solutions as described later. The Kevlar fiber is an array of molecules oriented parallel to each other like a package of uncooked spaghetti. This orderly, untangled arrangement of molecules is described as a crystalline structure. Crystallinity is obtained by a manufacturing process known as spinning, which involves extruding the molten polymer solution through small holes. When PPD-T solutions are extruded through a spinneret and drawn through an air gap during fiber manufacture, the liquid crystalline domains can orient and align in the flow direction. Kevlar can acquire a high degree of alignment of long, straight polymer chains parallel to the fiber axis. The structure exhibits anisotropic properties, with higher strength and modulus in the fiber longitudinal direction than in the axial direction. The extruded material also possesses a febrile structure. This structure results in poor shear and compression properties for aramid composites. Hydrogen bonds form between the polar amide groups on adjacent chains and they hold the individual Kevlar polymer chains together. It is shown in the following figure:
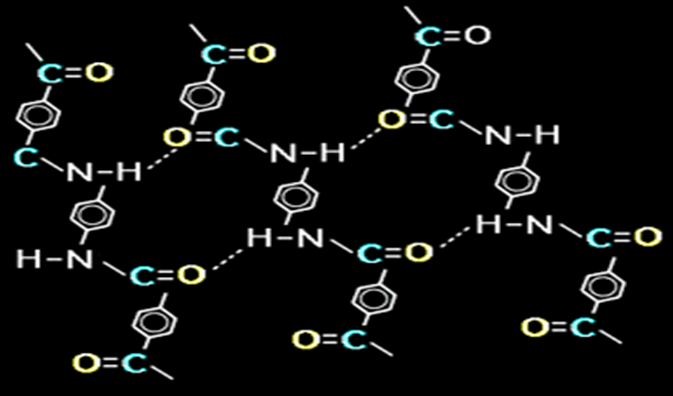
Figure 2: Hydrogen bonds form between the polar amide groups
Technical Properties:
Technical properties claimed by Para-Aramid Fiber can be summarized as follows:
* High strength-to-weight ratio.
* Low ductility.
* High modulus of rigidity (structural rigidity).
* Low electrical conductivity.
* High chemical resistance.
* Low coefficient of thermal expansion.
* High toughness (work-to-break).
* Excellent dimensional stability.
* Low machinability.
* Flame retardant, self-extinguishing.
Advantages and Disadvantages of Aramid Fiber:
Advantages are:
- It has the lowest specific gravity.
- It has the highest tensile strength-weight ratio.
- Only fiber for structural application.
Disadvantages are:
- Low compressive strength.
- Difficult to machine (Low machinability).
Applications of the Para-Aramid Fibers:
Because of its chemical nature and linearly oriented polymer structure, para-aramid fiber claims an excellent combination of physical properties. High tensile strength, high stiffness and damage tolerance, and high thermal stability with self-extinguishing properties (para-aramid fiber does not melt), make kevlar fibers ideal for a huge range of demanding applications. These exceptional properties, particularly its high strength-to-weight ratio, temperature resistance, and versatility, are thought responsible for para-aramid fiber being used in a huge range of demanding applications ranging from deep sea umbilical lines and premium sports goods to high-performance structural composites in boat hulls, aircraft components, and high-performance cars.
The para-aramid fiber is suggested to be used in the flywheel of a commuter car because the para-aramid fiber glass flywheel has a greater strength-to-weight ratio so that it can spin much faster than flywheels made of other materials which would shatter.
Para-aramid fiber is now being used to produce lightweight bulletproof body armor, and para-aramid vests are currently being worn by many of the country’s policemen. However, para-aramid fiber’s main chance seems to lie in markets created by the energy problem. Replacing heavier materials with Para-aramid fiber in airplanes, for instance, saves on fuel. The users also see para-aramid fiber as a replacement for steel cable on offshore oil rigs.
Para-aramid fiber and butacite laminated glasses make buildings safer, more durable, and more efficient. Kevlar is a lightweight, synthetic fiber, five times stronger than steel on an equal weight basis, used to protect buildings from blasts.
Wheels made with a single carbon fiber and para-aramid fiber spoke can run continuously from rim to rim, wrapping around, but not ending at the hub. The Kevlar and carbon fibers are manipulated into an airfoil shape and coated with a thin layer of clear thermoplastic resin. The spokes are then shaped to pass through the carbon-composite hub shell and span across the wheel. The spokes are joined to a standard rim with stainless steel fasteners and a standard alloy nipple.
Carbon fiber components are used in the manufacturing of motorcycles. Baxter, a textile engineering graduate of Clemson University, went on to invent Draggin’ Jeans, which use 100% para-aramid fiber in denim jeans. About 11 inches of the protective fabric is used in the knees, seam to seam, and the entire rear is covered with it.
A comprehensive list of Para-aramid fiber applications includes:
•Adhesives and sealants — Thixotropes;
•Ballistics and defense — Anti-mine boots, cut-resistant gloves, composite helmets, and bullet- and fragmentation-resistant vests;
•Belts and hoses — Automotive heating/cooling systems, industrial hoses, and automotive and industrial synchronous and power transmission belts;
•Composites — Aircraft structural body parts and cabin panels, boats, and sporting goods;
•Fiber optic and electromechanical cables — Communications and data transfer
cables; ignition wires; and submarine, aerostat, and robotic tethers;
•Friction products and gaskets —Asbestos replacement, automotive and industrial gaskets for high-pressure/high-temperature environments; brake pads; and clutch linings;
•Protective apparel — Boots; chain-saw chaps; cut-resistant industrial gloves; helmets (both for firefighters and consumer bicyclists); and thermal- and cut-protective aprons, sleeves, etc.;•Tires — Aircraft, automobiles, off-road, race vehicles, and trucks;
•Ropes and cables — Antennae guy wires, fishing line, industrial and marine utility ropes, lifting slings, mooring and emergency tow lines, netting and webbing, and pull tapes.
PROCESS DESCRIPTION
Most synthetic and cellulosic manufactured fibers are manufactured by “extrusion” — forcing a thick, viscous liquid (about the consistency of cold honey) through the tiny holes of a device called a spinneret to form continuous filaments of semi-solid polymer.
In their initial state, the fiber-forming polymers are solids and therefore must be first converted into a fluid state for extrusion. This is usually achieved by melting, if the polymers are thermoplastic synthetics (i.e., they soften and melt when heated), or by dissolving them in a suitable solvent if they are non-thermoplastic cellulosics. If they cannot be dissolved or melted directly, they must be chemically treated to form soluble or thermoplastic derivatives. Recent technologies have been developed for some specialty fibers made of polymers that do not melt, dissolve, or form appropriate derivatives. For these materials, the small fluid molecules are mixed and reacted to form the otherwise intractable polymers during the extrusion process.
The spinnerets:
The spinnerets used in the production of most manufactured fibers are similar, in principle, to a bathroom shower head (see Figure 3). A spinneret may have from one to several hundred holes. The tiny openings are very sensitive to impurities and corrosion. The liquid feeding them must be carefully filtered (not an easy task with very viscous materials) and, in some cases, the spinneret must be made from very expensive, corrosion-resistant metals. Maintenance is also critical, and spinnerets must be removed and cleaned regularly to prevent clogging.
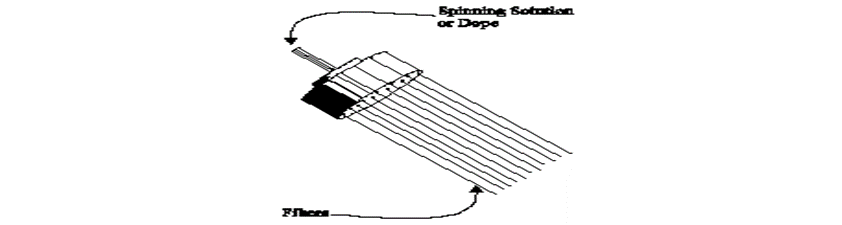
Figure 3: Spinnerets
As the filaments emerge from the holes in the spinneret, the liquid polymer is converted first to a rubbery state and then solidified. This process of extrusion and solidification of endless filaments is called spinning, not to be confused with the textile operation of the same name, where short pieces of staple fiber are twisted into yarn. There are four methods of spinning filaments of manufactured fibers: wet, dry, melt, and gel spinning. Table 1 lists the different types of spinning methods with fiber and gel spinning. Table 1 lists the different types of spinning methods with the fiber types produced by each method.
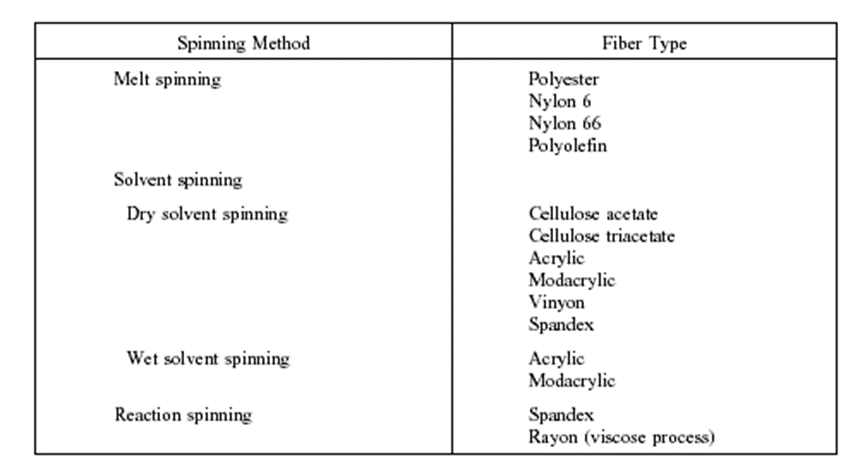
Table 2: Types of spinning methods and fiber types produced.
Types of Spinning Methods
(1) Wet Spinning:
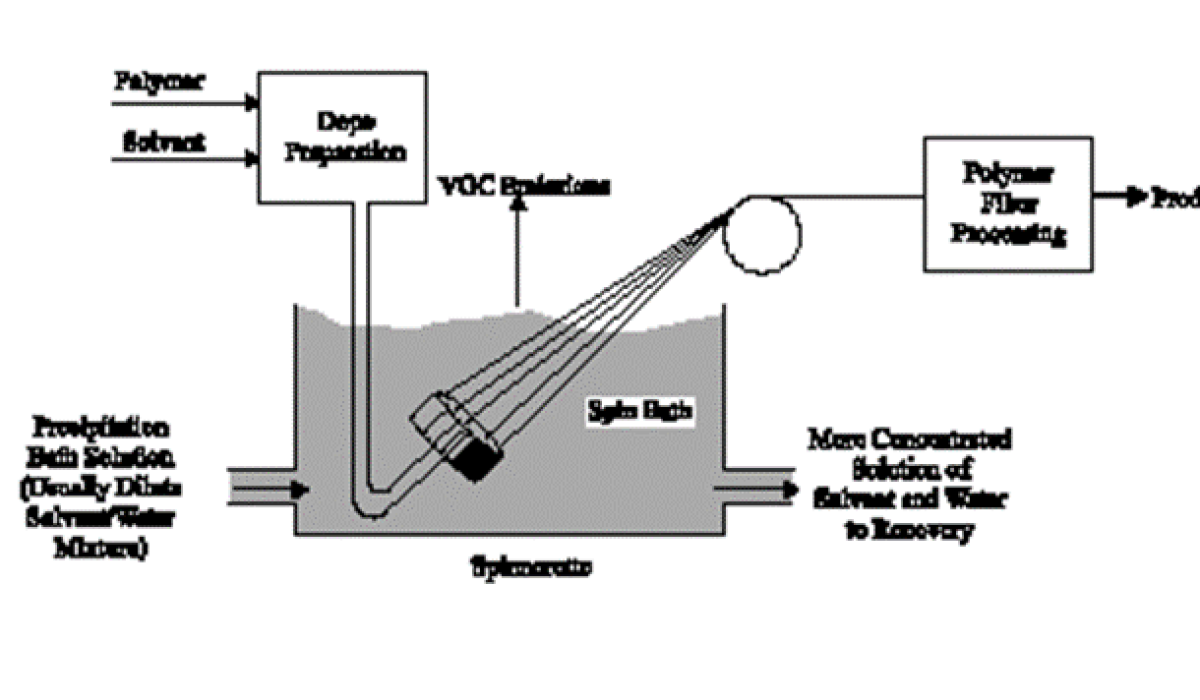
Wet spinning is the oldest process. It is used for fiber-forming substances that have been dissolved in a solvent. The spinnerets are submerged in a chemical bath and as the filaments emerge they precipitate from solution and solidify.
The process begins by dissolving polymer chips in a suitable organic solvent, such as dimethylformamide (DMF), dimethylacetamide (DMAc), or acetone, or in a weak inorganic acid, such as zinc chloride or aqueous sodium thiocyanate. In wet spinning, the spinning solution is extruded through spinnerettes into a precipitation bath that contains a coagulant (or precipitant) such as aqueous. Because the solution is extruded directly into the precipitating liquid, this process of making fibers is called wet spinning. Acrylic, rayon, aramid, modacrylic, and spandex can be produced by this process.
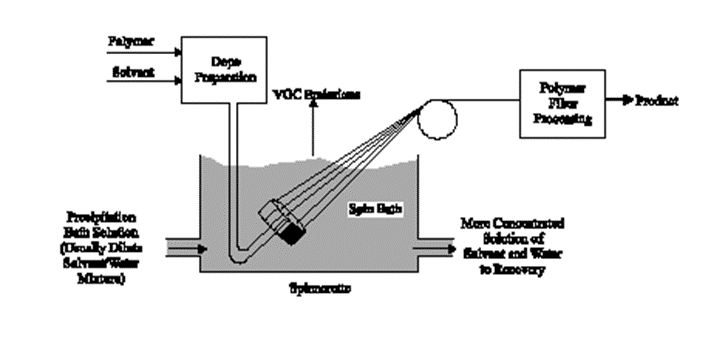
Figure 4: Wet spinning
(2) Dry Spinning:
Dry spinning is also used for fiber-forming substances in solution. However, instead of precipitating the polymer by dilution or chemical reaction, solidification is achieved by evaporating the solvent in a stream of air or inert gas. The filaments do not come in contact with a precipitating liquid, eliminating the need for drying and easing solvent recovery. The dry spinning process begins by dissolving the polymer in an organic solvent. This solution is blended with additives and is filtered to produce a viscous polymer solution, referred to as “dope”, for spinning. The polymer solution is then extruded through the spinnerets as filaments into a zone of heated gas or vapor. The solvent evaporates into the gas stream and leaves solidified filaments, which are further treated using one or more processes (See Figure 5.)
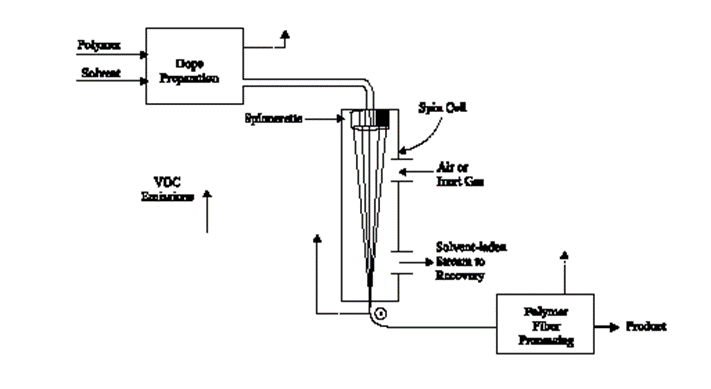
Figure 5: Dry spinning This process may be used for the production of acetate, triacetate, acrylic, modacrylic, PBI, spandex, and vinyon.
(3) Melt Spinning
In melt spinning, the fiber-forming substance is melted for extrusion through the spinneret and then directly solidified by cooling. Nylon, olefin, polyester, saran, and sulfur are produced in this manner.
Melt spinning uses heat to melt the polymer to a viscosity suitable for extrusion. This type of spinning is used for polymers that are not decomposed or degraded by the temperatures necessary for extrusion. Polymer chips may be melted by several methods. The trend is toward melting and immediate extrusion of the polymer chips in an electrically heated screw extruder. Alternatively, the molten polymer is processed in an inert gas atmosphere, usually nitrogen, and is metered through a precisely machined gear pump to a filter assembly consisting of a series of metal gauges interspersed in layers of graded sand. The molten polymer is extruded at high pressure and constant rate through a spinneret into a relatively cooler air stream that solidifies the filaments. Lubricants and finishing oils are applied to the fibers in the spin cell. At the base of the spin cell, a thread guide converges the individual filaments to produce a continuous filament yarn, or a spun yarn, that typically is composed of between 15 and 100 filaments. Once formed, the filament yarn either is immediately wound onto bobbins or is further treated for certain desired characteristics or end-use.
Melt-spun fibers can be extruded from the spinneret in different cross-sectional shapes (round, trilobal, pentagonal, octagonal, and others). Trilobal-shaped fibers reflect more light and give an attractive sparkle to textiles. Pentagonal-shaped and hollow fibers, when used in carpet, show less soil and dirt. Octagonal-shaped fibers offer glitter-free effects. Hollow fibers trap air, creating insulation and providing loft characteristics equal to, or better than, down.
(4) Gel Spinning
Gel spinning is a special process used to obtain high strength or other special fiber properties. The polymer is not in a true liquid state during extrusion. Not completely separated, as they would be in a true solution, the polymer chains are bound together at various points in liquid crystal form. This produces strong inter-chain forces in the resulting filaments that can significantly increase the tensile strength of the fibers. In addition, the liquid crystals are aligned along the fiber axis by the shear forces during extrusion. The filaments emerge with an unusually high degree of orientation relative to each other, further enhancing strength. The process can also be described as dry-wet spinning since the filaments first pass through the air and then are cooled further in a liquid bath. Some high-strength polyethylene and aramid fibers are produced by gel spinning.
DETAIL MANUFACTURING OF HIGH-STRENGTH PARA-ARAMID FIBER FIBERS
Although the specific details of the manufacturing of aramid fibers remain proprietary secrets, it is believed that the processing route involves solution polycondensation of diamines and diacid halides at low temperatures.
Effects of the Crystallinity of Para-aramid Fiber
The most important point is that the starting spinnable solutions that give high strength and high modulus fibers have liquid crystalline order. Various states of polymer in solution depend on the type of polymer chain. Two-dimensional, liner, flexible chain polymer in solution is called random coils. If the polymer chain can be made of rigid units, that is, rod-like, they can be represented like a random array of rods. Any associated solvent may contribute to the rigidity and to the volume occupied by each polymer molecule. With increasing concentration of rod-like molecules, one can dissolve more polymers by forming regions of partial order, that is, regions in which the chains form a parallel array. This partially ordered state is called a liquid crystalline state. When the rod-like chains become approximately arranged parallel to their long axes but their centers remain unorganized or randomly distributed, we have what is called a nematic liquid crystal. It is this kind of order that is found in the extended chain polyamides.
Liquid crystal solutions, because of the presence of the ordered domains, are optically anisotropic, that is birefringent. The parallel array of polymer chains in the liquid crystalline state becomes even more ordered when these solutions are subjected to shear. It is this inherent property of liquid crystal solutions that is exploited in the manufacture of aramid fibers (trade name of para-aramid fiber). The characteristic fibrillar structure of aramid fibers is due to the alignment of polymer crystallites along the fiber axis.
Spinning Solvent
Organic Fibers Researchers at DuPont discovered a spinning solvent for polyp-benzamide (PBA) and were able to dry spin quite strong fibers from tetramethylurea-LiCI solutions. This was the real breakthrough. The modulus of these spun organic fibers was greater than that of glass fibers. P-oriented rigid diamines and dibasic acids give polyamides that yield, under appropriate conditions of solvent, concentration, and polymer molecular weight, the desired nomadic liquid crystal structure. One would like to have, for any solution spinning process a high molecular weight to obtain improved mechanical properties, a low viscosity to ease processing conditions, and a high polymer concentration to achieve a high yield. For para-aramid, poly p-phenyleneterephthalamide (PPD-T), trade name Para-aramid fiber, the nematic liquid crystalline state is obtained in 100% sulfuric acid at a polymer concentration of about 20%. The polymer solution is often referred to as the dope. The various spinning processes available are classified as dry, wet, and dry jet-wet spinning processes (mentioned earlier).
Dry Jet Wet Spinning Method
For aramid fibers, the dry jet-wet spinning method is employed. It is believed that solution-polycondensation of diamines and diacid halides at low temperatures (near 00C) gives the aramid forming polyamides. Low temperatures inhibit product generation and promote linear polyamide formation. The resulting polymer is pulverized, washed, and dried. This is mixed with a strong acid (e.g., concentrated sulphuric acid) and extruded through spinnerets at 100 0C through about 1 cm air layer to cold water (0-4 0 C). The fiber precipitates in the air gap and the acid is removed in the coagulation bath. The spinneret capillary and air gap cause rotation and alignment of the domains resulting in highly crystalline and oriented as-spun fibers. At the end of this process, the Para-aramid fiber is produced.
CONCLUSION
In this essay, it is shown that fibers are formed by forcing a viscous fluid or solution of the polymer through the small orifices of spinnerets and immediately solidifying or precipitating the resulting filaments. This prepared polymer may also be used in the manufacture of other non-fiber products such as the enormous number of extruded plastic and synthetic rubber products.
(The essay is from the internet, If there is any infringement, please contact us to delete it.)